Energy storage system (ESS) is an important technology in the modern energy landscape, enabling the conversion of electricity into storable forms of energy since electricity in its original form cannot be stored.
With the growing adoption of renewable technologies like wind and solar, which provide intermittent power supply, and the inefficiencies associated with traditional power grids, the need for robust energy storage systems has become more critical than ever.
This blog post explores various energy storage technologies, highlighting their benefits and uses, and underscores the importance of ESS in fostering a sustainable and efficient energy future.
Also Read: Biomass Energy Explained | Resources, benefits & Tech
What is Energy Storage System
Energy Storage System (ESS) convert electrical energy from the power grid into a storable form, which you can later transform back into electrical energy when needed.
These systems store various forms of energy, such as electrical, thermal or mechanical, allowing you to use this stored energy during periods of higher demand, higher power production costs or insufficient generation resources.
ESS also addresses the challenges faced by modern power supply systems by enhancing the reliability, stability and power quality of both traditional power grids and highly variable Renewable Energy Sources (RES) and Distributed Energy Resources (DERs).
ESS address the following main issues in the power supply system:
- ESS match electricity supply with fluctuating demand.
- They facilitate the trading of stored energy, optimizing market operations.
- ESS maintain grid frequency stability, crucial for grid health.
- They alleviate congestion and support the grid during peak loads.
- ESS ensure consistent voltage levels, improving power quality.
- They provide backup power to maintain supply during sudden outages.
- ESS manage both active and reactive power, enhancing overall grid performance.
Also Read: Guide to Geothermal Energy | How Earth’s Power is Harnessed?
Basic Components of Energy Storage System
In an AC power system, electrical energy cannot be stored directly. Instead, AC energy is stored by converting it into other forms like kinetic, electromagnetic, electrochemical or potential energy.
The main elements of an Energy Storage System (ESS) include:
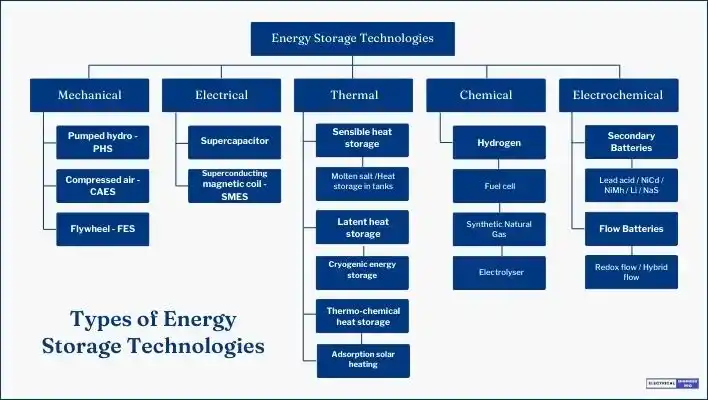
- Storage form/type: This component offers the means to store energy for future use, such as batteries, Pumped Hydro Energy Storage, Flywheel Energy Storage Systems, Supercapacitor Energy Storage, Thermal Energy Storage, Compressed Air Energy Storage and Superconducting Magnetic Energy Storage.
- Power Conversion Unit (PCU): This equipment converts stored energy into a usable form, typically involving inverters and rectifiers.
- Control System: This system manages the entire energy storage system, ensuring optimal performance and efficiency.
- Charging Unit: This component facilitates the flow of energy from the electrical system to the storage medium, ensuring proper energy intake and storage.
- Discharging Unit: This component allows the flow of stored energy from the storage medium to the load when required, ensuring energy availability during peak demand or other needs.
- Monitoring and Control Systems: These tools and software provide real-time monitoring, control and diagnostics of the ESS to ensure reliable and efficient operation.
- Safety and Protection Systems: These mechanisms ensure the safe operation of the ESS, including fire suppression, ventilation and fault detection systems.
Also Read: 8 Latest Trends in Electrical Engineering
Energy Storage Technologies
We can divide energy storage technologies into two categories: developed/mature technologies, which are currently available, and emerging technologies, which are not matured enough to be deployed on a larger scale and are anticipated to become available in the coming years.
Developed Technologies
Developed technologies have reached a high level of technological maturity and are currently deployed, widely used in various applications, including grid storage, electric vehicles, consumer electronics and industrial uses..
They have proven reliable and have well-established performance records. Companies readily supply these technologies to the market with well-established supply chains, benefiting from economies of scale that generally make them more cost-effective.
Extensive data on their performance, durability, and efficiency allows users to make informed deployment decisions. These technologies comply with existing regulatory standards and have gained broad market acceptance, supported by industry standards and certification processes that ensure their safety and reliability.
Examples
- Lithium-ion Batteries
- Pumped Hydro Energy Storage (PHES)
- Flywheel Energy Storage Systems (FESS)
- Compressed Air Energy Storage (CAES)
- Lead-acid Batteries
- Molten Salt Thermal Energy Storage (TES)
- Sodium-sulfur Batteries (NaS)
- Superconducting Magnetic Energy Storage (SMES)
- Vanadium Redox Flow batteries
Emerging Technologies
Researchers and developers are actively working on emerging technologies, which are in various stages of research, development and early-stage deployment. They are optimizing these technologies for performance, efficiency, cost, and scalability. Although these technologies have demonstrated potential, they lack the extensive track record of developed technologies.
Currently, companies do not widely offer these emerging technologies commercially, or they are only available in limited quantities. Higher costs often result from smaller production scales and ongoing development efforts. Limited data on long-term performance and reliability makes it harder to predict their full potential and lifecycle costs.
Additionally, developers are still navigating regulatory hurdles and establishing industry standards for these technologies. Market acceptance is in the early stages, with ongoing efforts to prove their viability and secure investment.
Examples:
- Solid-state Batteries
- Flow Batteries
- Advanced Supercapacitors
- Hydrogen Energy Storage
- Gravity-based Energy Storage
- Hybrid Energy Storage Systems
Also Read: Wind Energy 101: Explore the Basics of a Sustainable Future
Types of Energy Storage Technologies
Energy storage technologies enable the conversion of electricity into more stable, storable energy forms, intending to transform it back into electricity as needed. These technologies, including mechanical energy storage, chemical energy storage, electrochemical energy storage and thermal energy storage, accomplish this conversion process.
Mechanical Energy Storage
Pumped Hydro Energy Storage (PHES)
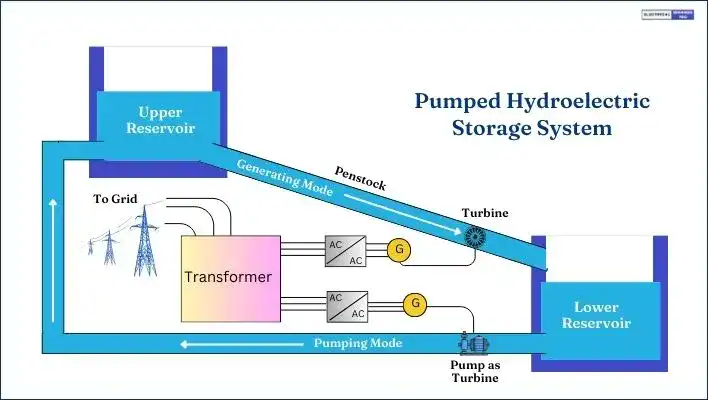
In Pumped Hydro Energy Storage (PHES), electricity is harnessed from the potential energy of a water body located at a relatively high elevation. The water is directed downhill from the upper reservoir through a pipe into a hydroelectric generator for storing it in the lower reservoir during peak hours.
During off-peak periods, water is pumped back up to replenish the upper reservoir, with the power plant serving as a load in the power system.
The PHES system comprises two large water reservoirs, an electric machine (motor/generator), and a reversible pump-turbine group or separate pump and turbine. This system can be activated within minutes, and its operation depends on the volume of stored water.
PHES facilities are typically sized up to 4000 MW and operate with efficiencies ranging from 76% to 85%, depending on the design. Pumped hydro plants boast long lifespans, lasting approximately 50-60 years.
As a general guideline, a reservoir with a diameter of one kilometer, a depth of 25 meters, and an average head of 200 meters would contain sufficient water to generate 10,000 MWh.
Compressed Air Energy Storage (CAES)
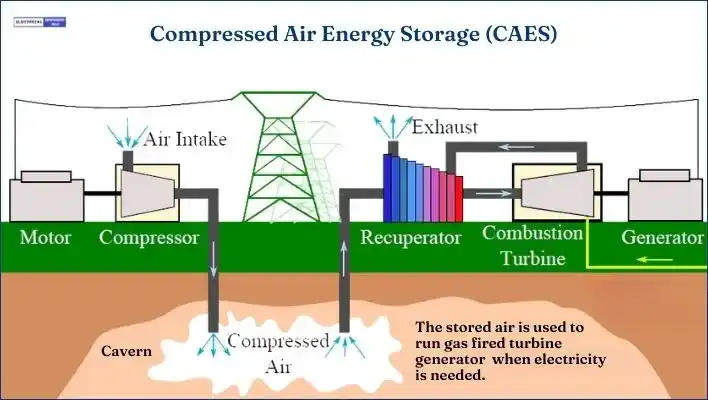
In Compressed Air Energy Storage (CAES), electricity is used to compress air. The compressed air is stored in either underground structures or above-ground systems of vessels or pipes. Common underground storage options include caverns, aquifers, and old mines.
When needed, the compressed air is mixed with natural gas and then ignited to expand within a modified gas turbine.
Different types of Compressed Air Energy Storage (CAES) exist, such as advanced adiabatic CAES, liquid air energy storage, supercritical CAES, isothermal CAES, and underwater CAES.
The advantages of CAES include large-scale storage, high discharge rates, and fast response. However, it also causes pollution through combustion and experiences efficiency losses.
Flywheel Energy Storage Systems (FESS)
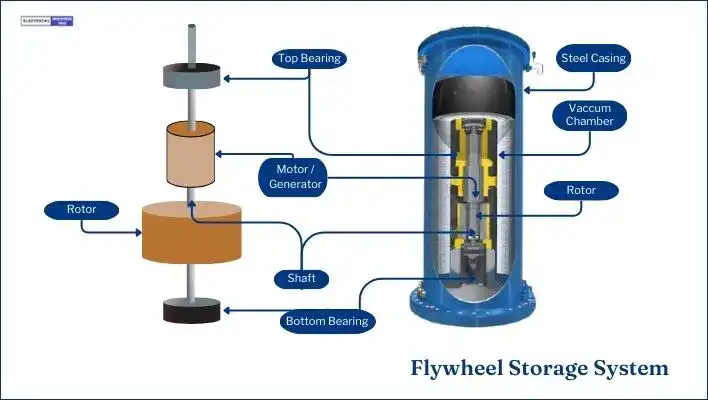
Flywheel Energy Storage Systems (FESS) harness energy by storing the angular momentum of a spinning mass known as a rotor. The system stores this energy in the form of kinetic energy, acquired through the exertion needed to set the mass spinning.
With the help of controls and power conversion systems, a flywheel system converts this kinetic energy into alternating current (AC) power.
The essential components of a flywheel include the rotating body or cylinder, typically composed of a rim attached to a shaft, housed within a compartment. Bearings and a transmission device, often a motor/generator affixed to the stator, support this mechanism.
Electric motors accelerate the flywheel, increasing its rotational speed and storing energy in the form of kinetic energy.
When electricity is needed, the flywheel’s rotational energy is converted back into electrical energy. This is usually achieved by reversing the process: slowing down the flywheel using a generator, which converts the rotational energy into electrical energy.
Key attributes of FESS include rapid response times, the ability to swiftly deliver substantial power, extended lifecycle, and minimal environmental footprint. However, challenges such as self-discharge rates and high initial costs currently hinder widespread adoption of this technology.
Electrical Energy Storage
Superconducting Magnetic Energy Storage (SMES)
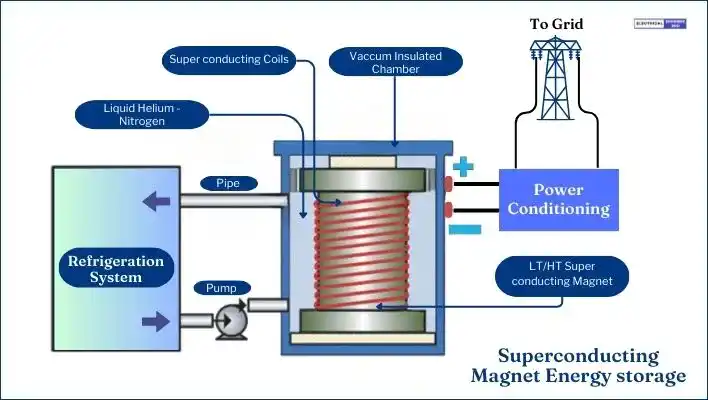
Superconducting magnetic energy storage (SMES) systems function based on electrodynamic principles. The electrodynamic principle refers to the fundamental laws and concepts governing the interaction between electric currents and magnetic fields.
In the context of SMES systems, a superconductor when cooled at extremely low temperatures, it causes the superconductor to exhibit zero electrical resistance and expel magnetic fields, a phenomenon known as the electrodynamic effect. This effect allows for the efficient storage of energy in the magnetic field generated by a DC current flowing through a superconducting coil.
SMES systems store electrical energy in a magnetic field generated by direct current (DC) flowing through a superconducting coil. This coil, which contains niobium-titanium (NbTi) filaments, operates at a superconducting critical temperature of approximately -270°C, ensuring nearly zero internal resistance.
The main component of an SMES system is the superconducting coil. Other parts include power conditioning equipment and a refrigeration system that uses cryogenic cooling.
These systems can rapidly charge and discharge, with a response time of just a few milliseconds. Despite their long operational life, their major drawback is the high cost associated with both initial setup and ongoing operation.
Supercapacitors
Supercapacitors, also known as ultra-capacitors, pseudo-capacitors, electric double-layer capacitors or gold capacitors, lead the latest advancements in electrical energy storage. Unlike conventional capacitors, supercapacitors store significantly larger amounts of energy.
Supercapacitors operate as an electrostatic type of energy storage system, storing energy in the form of an electric field. Their design features two separate porous electrodes immersed in a liquid electrolyte, with a separator placed between these electrodes to facilitate electrostatic energy storage.
Supercapacitors offer key advantages, including extremely fast response times measured in milliseconds, long lifespans, and high efficiency. However, they face challenges such as operating temperature limits, high costs, and lower energy density.
Thermal Energy Storage (TES)
Thermal Energy Storage (TES) allows the storage of heat and cold for later use, and is also referred to as heat or cold storage. TES can be categorized into two types:
- Low-Temperature TES (LT-TES)
- High-Temperature TES (HT-TES)
Low-Temperature TES (LT-TES)
LT-TES operates below 200°C and has been extensively researched and developed. These systems are typically used in building heating and cooling applications, solar cooking, solar water boilers, and air heating systems. LT-TES can be further divided into:
- Cryogenic Energy Storage
- Aquiferous LT-TES
High-Temperature TES (HT-TES)
HT-TES systems are primarily utilized in renewable energy technologies, waste heat recovery, and thermal power systems. Thermal energy in HT-TES can be stored through cryogenic, sensible heat, latent heat, or thermochemical methods. HT-TES includes:
- Sensible Heat TES
- Latent Heat of Fusion TES
- Concrete Thermal Storage
TES is widely used for generating electrical energy from heat engines and for load shifting.
Example: Pumped Thermal Energy System (PTES)
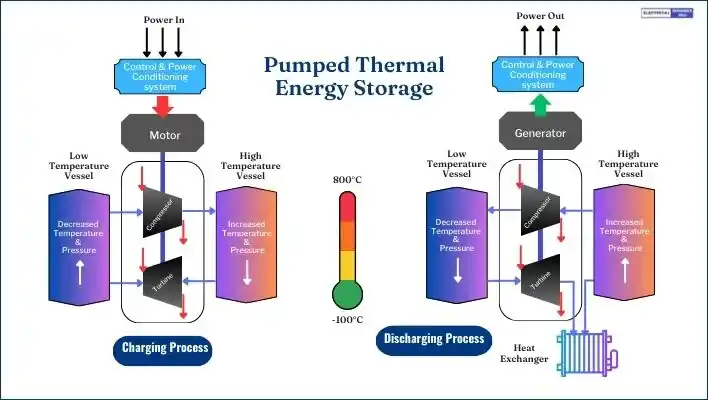
A pumped thermal energy system (PTES) is a type of TES. In this arrangement, a compressor and a turbine are coupled to an electrical machine that functions as both a motor and a generator for the processes of charging and discharging.
The system uses high-temperature and low-temperature chambers connected through pipes for heat exchange. These chambers are filled with argon gas, which heats and cools more quickly than air.
During the charging process, the compressor raises the temperature and pressure of the argon, preparing it for storage in the high-temperature chamber. While discharging, the process reverses, and the turbine converts the stored energy back to electricity.
Electrochemical Energy Storage System
In electrochemical energy storage systems, chemical energy is converted to electrical energy and vice versa.
Batteries are classified into two types: primary and secondary. Primary batteries are single-use and cannot be recharged, whereas secondary batteries can be recharged and reused.
Electrochemical energy storage batteries fall under the category of secondary storage devices, and most of these are technologically mature for practical applications.
A generalized diagram for the basic structure of the battery in Figure.
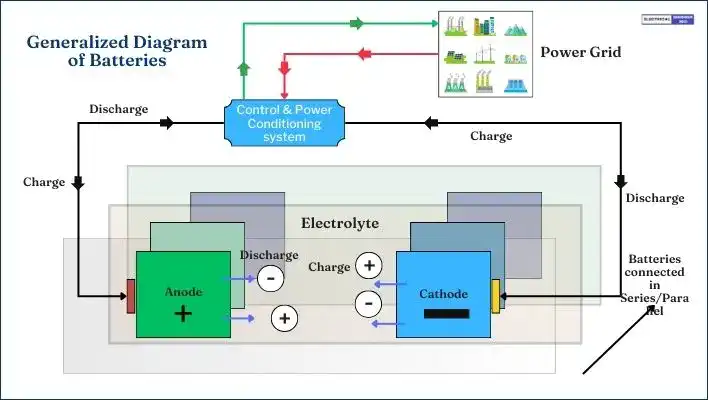
A battery is composed of multiple cells, each containing two electrodes, an anode and a cathode immersed in an electrolyte.
When an electrical load is connected to these electrodes, electrons flow through the closed circuit from one electrode to the other via the load and electrolyte.
Each cell typically has a terminal voltage of around 2V. Cells are connected in series or parallel configurations to achieve the desired voltage and current levels.
Six types of secondary batteries which are commonly used and have matured technologies are discussed below,
Lead acid battery (LA)
Since their commercial introduction, lead-acid batteries have become the world’s most widely used rechargeable battery.
They feature a positive electrode made of lead dioxide (PbO2) and a negative electrode made of metallic lead (Pb). Both electrodes are designed with highly porous active material to maximize surface area. The electrolyte typically consists of a sulfuric acid solution.
Many applications still rely on lead-acid batteries because they offer low cost, reliability, technological maturity, long lifespan, and quick response times, especially in automobile systems.
Lithium Ion Batteries (LiB)
Lithium-ion batteries (LiB) are lightweight and have a high energy density, making them ideal for portable applications such as electric vehicles and electronic devices.
Various types exist based on their internal chemistry, including Lithium Cobalt Oxide (LCO), Lithium Titanate Oxide (LTO), Lithium Manganese Oxide (LMO), Lithium Iron Phosphate (LFP), Lithium Nickel Manganese Cobalt Oxide (NMC) and Lithium Nickel Cobalt Aluminum Oxide (NCA).
Manufacturers commonly use cobalt oxide for the positive electrode in most commercial lithium-ion cells. However, alternatives like LiMnO2 and LiMn2O4, which are based primarily on manganese oxide, are also available. The negative electrode typically consists of carbon, either in the form of graphite or an amorphous material with a high surface area.
These batteries offer several advantages: they are portable, lightweight, economical, and capable of fast charging and discharging cycles. However, they have a significant drawback in their poor heat handling capability.
Nickel Cadmium Battery
The battery’s positive electrode contains nickel hydroxide or nickel oxide hydroxide, while the negative electrode uses cadmium or cadmium hydroxide, with potassium hydroxide as the electrolyte.
Ni-Cd batteries provide high power density and efficiency, along with low internal resistance, enabling fast charging and discharging cycles. However, they suffer from disadvantages such as toxicity and high cost.
Sodium-sulfur Battery
Sodium-sulfur (NaS) batteries are a commercial energy storage technology used for electric utility distribution grid support, wind power integration and grid services. Their long discharge periods make them ideal for these grid services.
Like many other storage technologies, NaS batteries can respond quickly and precisely to grid needs, such as mitigating power quality events and responding to automatic generation control signals for area regulation.
NaS batteries stand out because they use solid electrolytes and molten phase electrodes. During discharge, molten sodium, acting as the negative electrode, releases electrons, while molten sulfur serves as the positive electrode.
Sodium ions travel through the solid electrolyte to the positive electrode. However, the high operating temperature and the use of pure sulfur pose risks, as sulfur can ignite when it contacts air or moisture.
Metal Air Battery (M-air)
Metal-Air battery systems operate through electrochemical charge and discharge reactions between a positive “Air Electrode” and a negative “Metal Electrode”.
The negative electrode typically comprises metals such as lithium (Li), zinc (Zn), aluminum (Al), iron (Fe), or sodium (Na). Meanwhile, the positive electrode combines a porous carbon material with a catalyst. The electrolyte, which can be aqueous or non-aqueous, plays a crucial role in systems like Li-Air batteries.
In this process, oxygen from the atmosphere penetrates the porous carbon electrode, where a catalyst facilitates its reduction. Simultaneously, the metal undergoes oxidation at the anode. A substantial portion of the cell’s volume accommodates the anodic material, contributing to the high energy densities characteristic of M-Air batteries.
Because of their scalability and high energy density, M-Air batteries find application in large-scale stationary energy storage. They prove especially valuable when integrated with renewable energy sources like wind or solar power, effectively addressing the intermittent nature of these energy sources.
Sodium Nickel Chloride Battery (NaNiCl)
Similar to Sodium Sulfur (NaS) batteries, Sodium Nickel Chloride (NaNiCl) batteries function at high temperatures. They operate within the normal temperature range and were primarily designed for use in electric vehicles (EVs) and hybrid electric vehicles (HEVs).
During the charging phase, NaCl salt and Ni undergo a chemical reaction, transforming into NiCl2 and molten Na. This process reverses during the discharging phase. One notable advantage of this reaction is the absence of any side reactions.
Ongoing research focuses on enhancing the performance of NaNiCl batteries. Researchers are developing advanced versions to achieve higher power densities for hybrid electric vehicles.
Additionally, they are working on creating high-energy variants suitable for storing renewable energy in applications such as load-leveling and industrial settings.
Flow Batteries
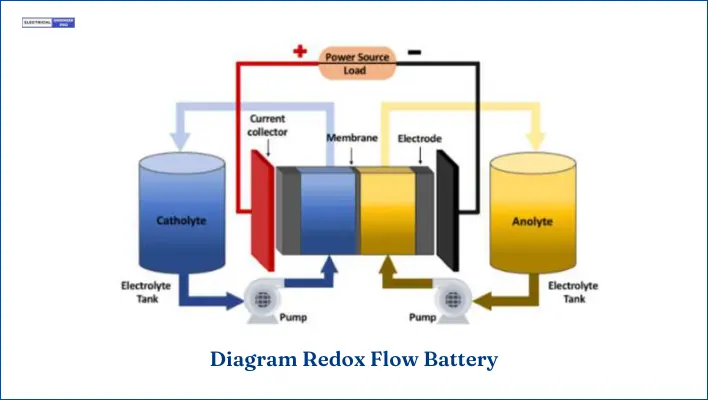
Flow batteries differ from previously discussed secondary batteries. It stores two soluble electrolytes in two separate tanks, which convert chemical energy to electrical energy through the electrochemical cell.
The energy density of a flow battery is determined by the size of its electrolyte tank, whereas the power density depends on the design of the cell.
Flow batteries are categorized into redox flow batteries and hybrid flow batteries. In a redox flow battery, electroactive material dissolves in a liquid electrolyte, while in a hybrid flow battery, one or more components are deposited. In a redox flow battery, the electroactive material is stored in separate tanks that circulate through a pump.
Flow batteries offer several significant advantages over other battery types. Their capacity can be increased by enlarging the tank, and since they lack electroactive material in the electrode, their chemical and physical properties remain stable, providing consistent and longer-lasting performance.
Various types of flow batteries exist, with at least three varieties currently available commercially: vanadium redox flow batteries, zinc-iron flow batteries, and zinc-bromine batteries. Additionally, variations such as zinc-iron flow batteries and hydrogen-bromine flow batteries are currently under development.
Chemical Energy Storage (CES)
Using chemical energy storage involves harnessing the energy stored in chemical bonds. Whenever chemicals undergo reactions, they release or absorb energy. Some reactions release a significant amount of energy as bonds break, which can be utilized to generate electricity.
Chemical compounds chosen for storage typically possess a higher energy density per unit volume compared to mechanical energy storage methods, these qualities make chemical compounds ideal for energy storage.
There are a variety of chemicals known for their energy storage capabilities. These include hydrogen, methane, various hydrocarbons, methanol, butanol, and ethanol.
Butanol and ethanol differ slightly as they are produced through the fermentation of biomass, making them unconventional electrical storage methods.
Hydrogen Energy Storage System (HESS)
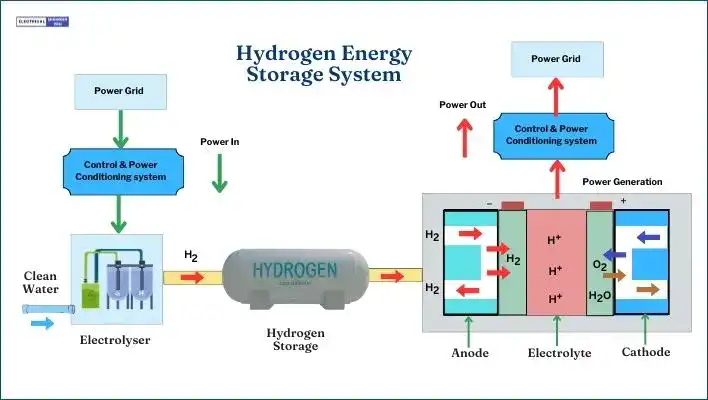
Hydrogen is a top choice for reducing our reliance on fossil fuels because it’s abundant and the simplest element available in nature. Because of these highlights, Hydrogen Energy Storage Systems (HESS) are getting attention for their ability to store energy effectively.
In this technology, excess electricity produced is used to split water into hydrogen and oxygen through electrolysis. The hydrogen is stored, and when needed, it’s converted back into electricity through a process called hydrogen oxidation. This conversion happens in fuel cells, which are like batteries fueled by hydrogen.
The main equation for this process is: 2H2 + O2 → 2H2O + Energy Production
Key parts of the system include the electrolyzer, fuel cell, hydrogen storage, and power conversion for electricity. The electrolyzer is crucial, splitting water into hydrogen and oxygen. The hydrogen is stored until it’s needed, then the fuel cells turn it back into electricity.
There are different types of fuel cells, like
- solid oxide fuel cell (SOFC)
- polymer electrolyte membrane fuel cell (PEMFC)
- alkaline fuel cell (AFC)
- phosphoric acid fuel cell (PAFC)
- molten carbonate fuel cell (MCFC)
- direct methanol fuel cell (DMFC)
HESS offers several advantages, including zero pollution, a Depth of Discharge (DoD) of up to 100% and minimal self-discharge rates (dependent on storage type). However, its drawbacks include relatively low efficiency (40-50%), high setup costs and limited security measures.
Uses and Benefits of Energy Storage Systems
According to US Energy Information Administration (EIA), following are the uses and benefits of Energy Storage Systems,
Balancing Grid Supply and Demand and Improving Quality and Reliability
- Energy storage systems (ESSs) help balance electricity supply and demand across various time scales.
- Fast response ESSs provide support to maintain electric grid frequency, ensuring power quality and reliability.
Peak Electricity Demand Shaving and Price Arbitrage Opportunities
- Charging during low-demand periods and discharging during high-demand periods flattens daily load curves.
- Reduces reliance on expensive reserve generation capacity, leading to lower wholesale electricity prices.
Storing and Smoothing Renewable Electricity Generation
- ESSs enhance the use of intermittent renewable energy sources like solar and wind.
- Enables responding to grid operator dispatch calls when direct generation is limited.
- Prevents wastage of excess renewable energy and supports off-grid systems.
Deferring Electricity Infrastructure Investments
- ESSs strategically placed on the grid manage growing demand without costly infrastructure upgrades.
Back-Up Power
- On-grid ESSs offer emergency back-up electricity during grid outages.
Reducing End-User Demand and Demand Charges
- Commercial and industrial consumers deploy on-site storage to reduce peak demand and associated charges.
- Supports utility demand-side management programs.
Integration with Microgrids
- ESSs integrated into microgrids provide various storage benefits and can isolate during grid interruptions.
References
- eia.gov/energyexplained/electricity/energy-storage-for-electricity-generation
- https://www.ucsusa.org/resources/how-energy-storage-works
- https://www.niti.gov.in
- https://www.energy.gov/eere/solar/solar-integration-solar-energy-and-storage-basics
- https://www.epa.gov/energy/electricity-storage
- Mushid, F. C.; Khan, M. F. A Survey on Energy Storage in Electric Power Systems & Its Applications in MV/LV Networks. Preprints 2023, 2023081901. https://doi.org/10.20944/preprints202308.1901.v1